The Pharma Manufacturing World Summit is a premier gathering of industry leaders, pharmaceutical manufacturing executives, and experts dedicated to exploring innovative strategies, emerging trends, and best practices in the dynamic world of pharmaceutical management.
PMWS gathers SVPs, VPs, and Directors of Manufacturing, Technical Operations, Quality, and Supply Chain, Technology Leaders, and Plant/Site/Facility Managers to share insights, foster innovation, and build invaluable connections. Dive into a thoughtfully curated environment that brings together thought leaders, allowing you to explore new ideas and engage in meaningful discussions. Join the conversation at PMWS and be part of a community dedicated to pushing the limits of pharmaceutical manufacturing success.


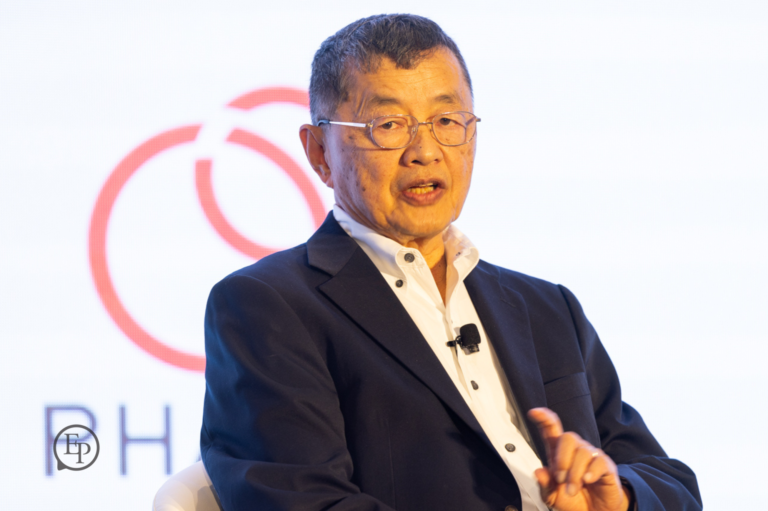











Year after year, we bring to the stage today's thought leaders and innovators

Pam Cheng joined AstraZeneca in 2015 as executive vice president of Global Operations and Information Technology (IT), guiding the company’s manufacturing, supply chain, procurement, and IT across 18 countries and leading a team of over 19,000. Under her leadership, AstraZeneca Global Operations has transformed significantly driving top performance across the business while delivering value back to the enterprise. The next phase of transformation includes the convergence of operational excellence and digital innovation, building the ‘Factory of the Future’.
Before AstraZeneca she spent 18 years in global manufacturing, supply chain, and commercial roles at Merck/MSD. As president of MSD China, she was responsible for MSD’s entire business in China—sales and marketing, commercial operations, and oversight of manufacturing and research and development. Prior to that, she was head of Global Supply Chain Management and Logistics for Merck and led the transformation of Merck supply chains across the global supply network. Before joining the Biopharmaceutical industry, she worked in engineering and project management roles at Universal Oil Products, Union Carbide Corporation, and GAF Chemicals.
In addition to her executive role at AstraZeneca, Pam is a nonexecutive director for the board at Smiths Group plc.
Ms. Cheng holds bachelor’s and master’s degrees in chemical engineering from Stevens Institute of Technology and an MBA in marketing from Pace University.
Pam Cheng
Executive Vice President, Global Operations & Information Technology
AstraZeneca


Melissa Seymour is executive vice president, Global Quality for Eli Lilly and Company.
Melissa previously served as the chief quality officer for Bristol Myers Squibb. In this role, she led the GPS Quality organization in setting the quality compliance strategy, implementation of quality processes and systems, and development of talent to ensure the highest level of quality and compliance. Before joining Bristol Myers Squibb, Melissa was also the chief quality officer at Biogen. She has had prior roles providing oversight for QC laboratories and spent several years as the vice president of Corporate Quality, with responsibility for global compliance and quality systems as well as in-market Quality.
Melissa earned a Bachelor of Science degree in Biological Sciences and Biochemistry from North Carolina State University and an Executive Master of Business Administration degree from Duke University. She has over 25 years of experience in the quality arena, including quality positions at Novo Nordisk and Glaxo Smith Kline.
Melissa has been involved in influencing regulatory guidance through her participation on non-profit Boards of the Parenteral Drug Association since 2016, currently serving as chair-elect; as well as Rx-360 consortium focused on supply chain security vis-à-vis public health concerns and patient safety. She has been an advocate for simplification of post approval change processes, participating in industry forums, writing articles and interacting with regulators. She holds certifications from ASQ as a Certified Quality Auditor, Quality Engineer and Quality Manager.
Melissa Seymour
Executive Vice President, Global Quality
Eli Lilly


Arleen Paulino, senior vice president, Manufacturing, leads Amgen’s commercial manufacturing organization. Prior to this role, Paulino served as vice president, Site Operations at Amgen Singapore Manufacturing from 2016 to 2018, where she led the team to the successful licensure of Amgen’s first Next-Generation Biomanufacturing plant.
Paulino joined Amgen in 2002 and over the years has held various positions with increasing responsibility in Process Engineering and Process Development. She was also the head of Clinical Operations and Development Supply Chain, where she was responsible for the end-to-end supply chain for the manufacture and delivery of clinical product to support Amgen’s global clinical trials.
She began her career in Operations at Genentech and later joined Immunex, where she held a variety of roles overseeing development and scale-up operations, contract manufacturing and technology transfer, as well as plant manager for the cell culture facility.
Paulino holds a Bachelor of Science degree in Biochemistry from Marquette University.
Arleen Paulino
Senior Vice President Global Manufacturing
Amgen


As Chief Technical Operations and Quality Officer, Dr. Collins is responsible for the technical development, quality, and supply of preclinical, clinical and commercial programs within Moderna’s portfolio.
Dr. Collins joined Moderna from Novartis, where he held roles of increasing responsibility over the last nearly 30 years, focused on pharmaceutical production and manufacturing, including roles serving as Head of Global Chemical Operations and Anti-Infectives and as Head of Global Chemical Operations.
Dr. Collins received his Bachelor of Science in Chemistry and his Ph.D. in Organic Chemistry from University College Cork, Ireland. He also conducted post-doctoral cancer research at Arizona State University.
Jerh Collins
Chief Technical Operations & Quality Officer
Moderna


Kathleen is a Biopharmaceutical leader with experience in small biotech and global Fortune 500 companies including bluebird bio, Intercept, BMS, Catalent, and now Biogen. She has developed and led site and HQ-based teams spanning Quality, IT, Product Development, and Supply Chain functions. She has deep expertise in managing Health Authority inspections and has tackled strategic and operational challenges, from global business process implementation to managing day-to-day operations. Kathleen enjoys solving complex problems, managing large organizations, and aligning teams on the most effective path forward that ensures compliance and enables the business.
Kathleen Munster
Chief Quality Officer
Biogen


Tim Maines joined Alnylam in August 2017 as the Global Head of Quality Assurance and Quality Control and was promoted to Chief Technical Operations and Quality Officer in April of 2023, holding over 35 years of experience. Prior to joining Alnylam, Tim was a member of the senior leadership teams at Ariad Pharmaceuticals, Omthera Pharmaceuticals, Astra Zeneca/MedImmune, GTC Biotherapeutics (formerly Genzyme Transgenics) and Stryker Biotech where he provided strategic input and independent quality and technical oversight of each company’s global GxP operations. In total, these experiences resulted with the commercialization and market expansion of thirteen novel medicines, including four FDA-approved therapies at Alnylam.
Tim holds a Bachelor of Science in Biology/Microbiology from Gannon University.
Timothy Maines
Chief Technical Operations & Quality Officer
Alnylam Pharmaceuticals


Steven Wesel serves as director of upstream process development at Forge Biologics and he has been with the company for over 4 years. He oversees the development and tech transfer of upstream gene therapy production processes, including cell banking, seed train, bioreactor scale up, transfection, and harvest of viral vectors. Steven has worked in upstream process development in biotechnology for the last thirteen years and in gene therapies for the last seven years. His experience prior to Forge includes leading upstream process development at Abeona Therapeutics, monoclonal antibody upstream process development at Gilead Sciences, and vaccine upstream process development at Merck. Steven’s expertise ranges from clonal selection to preclinical development through clinical manufacturing. He has worked with many scales, ranging from high throughput small scale Ambr15 (15 mL) bioreactors up to manufacturing scale 2000 L productions. He has a Master’s degree from Lehigh University and Bachelor’s from MIT in Chemical and Biological engineering. He also has an MBA from the University of North Dakota.
Steven Wesel
Director upstream process development
Forge Biologics


Charles L. Cooney is the Robert T. Haslam (1911) Professor of Chemical and Biochemical Engineering, Emeritus in the Department of Chemical Engineering at MIT and founding Faculty Director, Emeritus of the Deshpande Center for Technological Innovation. He has been involved as founder, advisor or board member of over 25 companies and currently sits on the Boards of Directors of Codiak Bioscience, Innovent Biologics (1801.HK), Levitronix Technologies, and is chairman of GreenLight Bioscience and Mitra RxDx. In addition, he is Trustee Emeritus of Boston Ballet, Advisor Emeritus of the Boston Symphony Orchestra and Trustee of the Leventhal Map Center. Other interests include: high altitude mountaineering (assents of Denali, Ama Dablam, Mont Blanc, Kilimanjaro, Huascaran). and antique map collecting.
Charles L. Cooney
Robert T. Haslam Professor of Chemical Engineering, Emeritus, and Faculty Director, Emeritus Deshpande Center for Technological Innovation
MIT


Dr. Joanne Beck is currently Chief Technology Officer at Abata Therapeutics. Prior to this, she served as Chief Technology Officer at Aerium Therapeutics and COO at Boston Pharmaceuticals, a private company developing candidates across multiple indications in cardiometabolic, oncology, and immune/inflammatory diseases. Prior to joining Boston Pharmaceuticals, she was Executive Vice President of Global Pharmaceutical Development and Operations at Celgene where she oversaw the company’s Product Development, Global Manufacturing Operations, Supply Chain, Engineering and Quality. Prior to Celgene, she was SVP of Pharmaceutical Development at Shire. Prior to Shire she held roles of increasing responsibility in Process Development at Genentech and Amgen and in Operations at Abbott’s Pharmaceutical and Vascular Divisions. Beck holds a BA in Chemistry from Lewis and Clark College, a Ph.D. in Biochemistry and Molecular Biology from University of Oregon Medical School and completed a postdoctoral fellowship in the department of Pharmaceutical Chemistry at the University of California, San Francisco. Dr. Beck serves on the board of directors for Orchard Therapeutics and Astria Therapeutics.
Joanne Beck
Chief Technical Officer
Abata Therapeutics


Judy joined Gilead Sciences as Vice President, Global Supply Chain in January 2024. She leads supply chain professionals responsible for end-to-end global supply chain strategy and execution to ensure uninterrupted supply of Gilead’s clinical product portfolio from trials through to launch. Close partnership with CMC functions including Product & Portfolio Strategy, CMC Regulatory Affairs, Technical Development, Manufacturing Operations and Quality Assurance will be critical part of her accountabilities. Judy is an accomplished healthcare supply chain professional with blended experiences in both big pharma and smaller, clinical-stage biotech companies. She is a visionary, solution-focused leader with a strong track record delivering best-in-class improvement strategies and strengthening portfolios. She has a passion for developing people to help them realize their potential and maximize their contributions.
Judy Yeh
Vice President, Global Clinical Supply Chain
Gilead Sciences

"*" indicates required fields
By completing and submitting this form, you agree to receive marketing emails from Executive Platforms Inc. You can opt-out at any time by utilizing the unsubscribe link provided at the bottom of each email. All data collected will be handled in accordance with our Privacy Policy and Terms of Use.
The Pharma Manufacturing World Summit 2023 gathered top pharmaceutical manufacturing leaders to share new ideas and collaborate on solutions that inspired a collective vision for change, shaping the future of the industry.
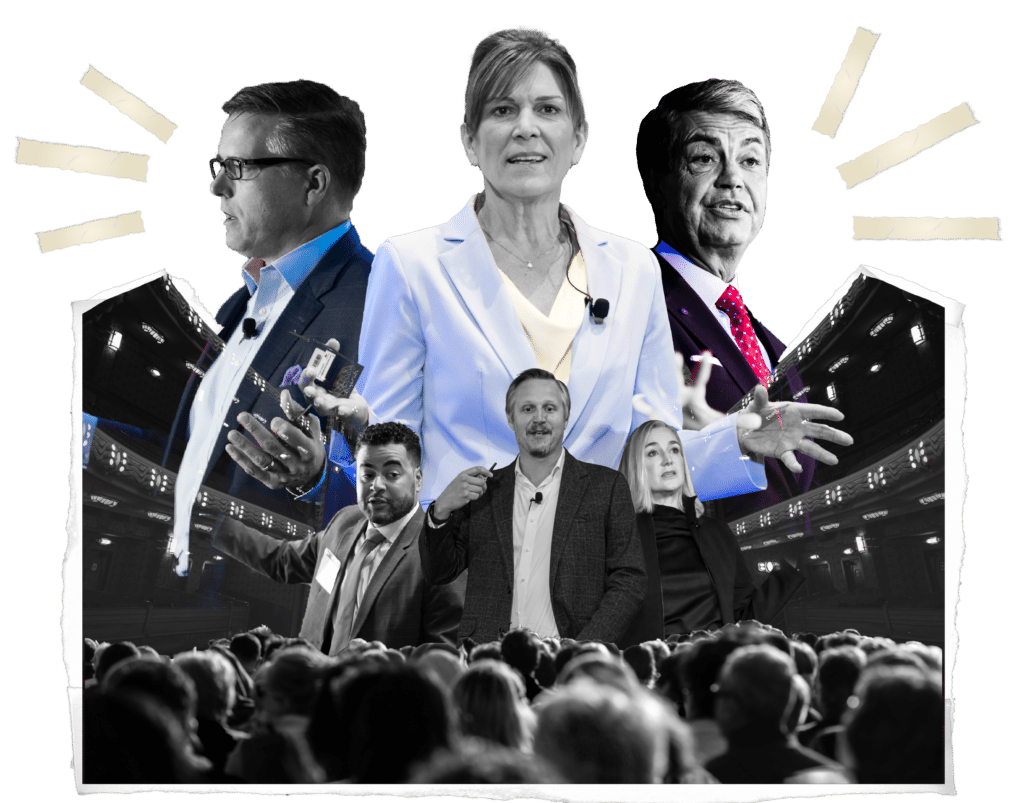
VP of Quality, INCOG BioPharma Services
Executive Director, Gilead Sciences
Head of Integration Management Office at Indivior
Director, Mfg. Sciences and Tech. at Resilience
Sr. Director Biotech TS/MS, Eli Lilly
Director, Supply Chain Strategic Planning, Merck
VP of Quality, INCOG BioPharma Services
Executive Director, Gilead Sciences
Head of Integration Management Office at Indivior
Director, Mfg. Sciences and Tech. at Resilience



































"*" indicates required fields
By completing and submitting this form, you agree to receive marketing emails from Executive Platforms Inc. You can opt-out at any time by utilizing the unsubscribe link provided at the bottom of each email. All data collected will be handled in accordance with our Privacy Policy and Terms of Use.
Notifications