A Decade Strong — Celebrating 10 Years of BMWS
A Decade Strong — Celebrating 10 Years of BMWS
The Biomanufacturing World Summit is a premier gathering of industry leaders, biomanufacturing executives, and experts dedicated to exploring innovative strategies, emerging trends, and best practices in the dynamic world of biomanufacturing.
BMWS gathers Senior Manufacturing Executives, Technical Operations Quality Executives, and Biomanufacturing Executives to share insights, foster innovation, and build invaluable connections. Dive into a thoughtfully curated environment that brings together thought leaders, allowing you to explore new ideas and engage in meaningful discussions. Join the conversation at BMWS and be part of a community dedicated to pushing the limits of biomanufacturing success.
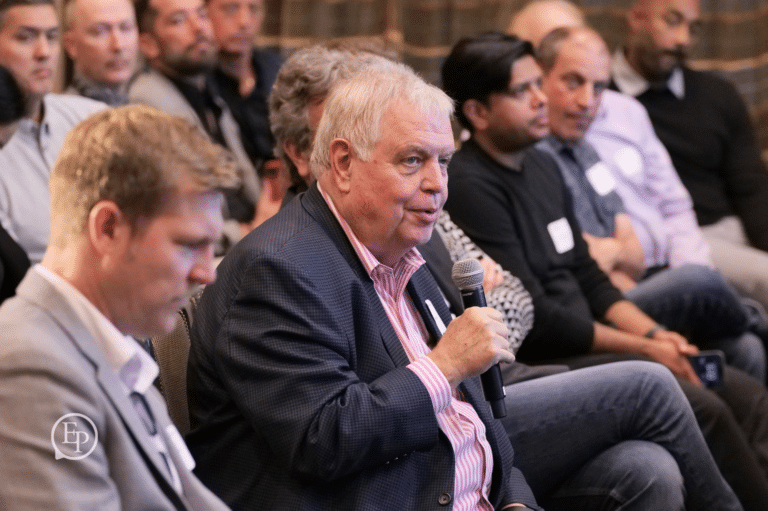
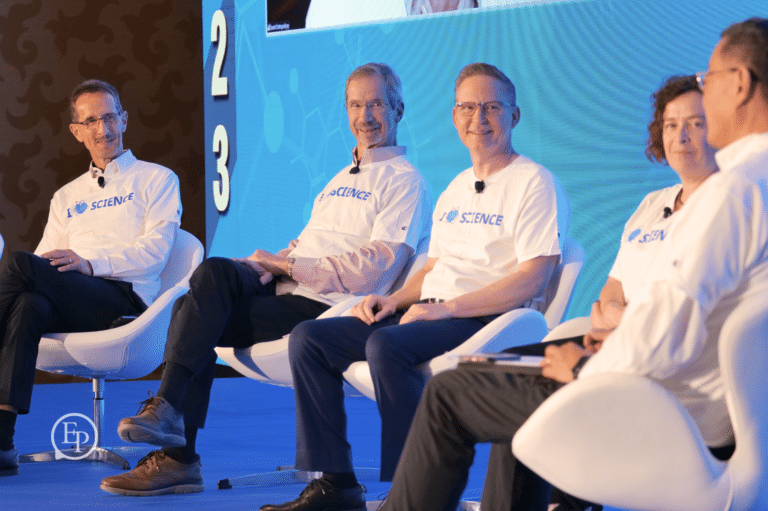










Year after year, we bring to the stage today's thought leaders and innovators

Karin began her career in the pharmaceutical industry at Bristol-Myers Squibb in their corporate global procurement organization. She quickly moved from procurement into global supply chain where she focused on business process improvements to enhance performance in areas such as product launch and commercialization. Following an assignment with global quality, Karin moved into manufacturing operations, leading two manufacturing sites. Leading a radiopharmaceutical site in Billerica, MA, Karin significantly improved product cost and site performance. During her tenor in Latina, Italy, where she led a chemical and drug product facility, Karin turned around the site’s performance in cost, quality and supply. After returning to the United States, Karin left BMS to join Becton-Dickenson leading global operations in Pre-Analytical Services, followed by Catalent Pharmaceuticals where she built their global Biologics capabilities while introducing operational excellence across the biologics and sterile operations network. In 2013 she joined Teva Pharmaceuticals, where she transformed a network of 25 sites in Canada, US, Latin America and Europe; later as COO, Global Operations she led network strategy and global manufacturing science & technology. Prior to rejoining Bristol Myers Squibb in 2023, she held the role SVP, Global Biologics & Sterile Operations at Merck & Co. for 4 years, where she built internal biologics and sterile manufacturing capabilities to supply importance products such as Gardasil and Keytruda as well as the growing biologics pipeline.
In her current role, EVP, Global Product Development & Supply, Karin is a member of the BMS executive committee reporting directly to Chris Boerner, BMS Chairman and CEO. In this role she has responsibility for product development, quality, supply chain, manufacturing operations, global facilities & engineering, and business analytics. Since rejoining BMS she has significantly improved operational performance and is building technical capabilities to deliver an increasingly complex portfolio of products across biologics, small molecule, cell therapy and radiopharmaceuticals.
She has enjoyed growing in her career while also building a family with her husband, Tim. Together they enjoy three daughters (Amber, Sarah and Kathryn) and their dog, Tucker.
She graduated from Rutgers University with her BA in Political Science/International Relations with a minor in German, and holds a Masters in Pharmaceutical & Device Law from Seton Hall University.
Karin Shanahan
EVP, Global Product Development & Supply
Bristol Myers Squibb

She leads a global, multicultural team across North & Latin America, Europe, and Asia responsible for all aspects of Supply Chain Operations and Value Chain Management (Product and Manufacturing strategies) at Merck. Her team is responsible for the supply chain management of Merck’s Human Health portfolio covering Small Molecules, Biologics, and Vaccines.
Bala started her career as an intern at Amazon.com, followed by start-up experiences and pivoting to Pharmaceutical Manufacturing. She began as a shop floor Technical Operations Engineer and, over the last 20 years, held roles of increasing responsibilities across the value chain in Manufacturing, Technical Operations, Engineering, LEAN/Six Sigma, and Supply Chain Management. Having led significant transformation and digital initiatives, she is also a D&I champion in the company and a champion for Merck’s ESG efforts to create sustainable and accessible supply chains.
Bala has a bachelor’s degree from the University of Madras, India, a master’s degree in electrical engineering from Southern Illinois University in Carbondale, and Executive education from Harvard Business School. Bala lives in Doylestown, Pennsylvania, with her husband and three kids (2 humans and a pug).
Bala Sreenivasan
SVP, Global Supply and Value Chain Management
Merck


Pascal leads Russell Reynolds’ operations in New York and Canada. He works closely with our healthcare clients around the globe, advising them on their talent strategy, including leadership assessment, succession planning, and the recruitment of directors, CEOs, and other senior executives.
Within the healthcare industry, Pascal specializes in biotech and pharma technical operations, including process development, manufacturing, quality, engineering, and supply chain leadership.
Pascal received his Bachelor of Engineering Physics degree from the Royal Military College of Canada and trained as an aerospace engineer with the Royal Canadian Air Force. He also earned master’s and doctorate degrees in business administration from Athabasca University in Canada, where he focused his research and dissertation on CEO succession. Pascal speaks fluent French and English.
Pascal Bécotte
Managing Director
Russell Reynolds


David Geoghegan is the Head of Quality Assurance and Validation for Spark Therapeutics, Inc, a part of the Roche portfolio of Gene therapy product development. Since joining Spark in 2021, he’s lead a team of scientists and professionals who are responsible for all aspects of Quality and Validation throughout the development lifecycle of gene therapy products, including non-clinical and clinical development programs as well as the first approved commercial gene therapy product. Recently, this team successfully hosted inspections of the Luxturna manufacturing facility in Philadelphia by FDA, PMDA, ANVISA, EMA and SFDA.
Prior to joining Spark, David worked for three years at Tmunity Therapeutics, Inc. as the Head of Quality and Validation. Tmunity worked to develop CAR-T cell therapies to treat solid tumor cancers. While there, he was a part of the core leadership team that built and validated a new state-of-the-art Viral Vector and Cell Therapy development and manufacturing facility. The quality systems that David implemented bridged the early development work done at an academic-based advanced therapy CMO to late-stage and commercial-ready in-house manufacturing and analytical operations.
Before joining Tmunity, Geoghegan worked over 30 years in various leadership roles within Operations. His roles included VP level roles in Manufacturing, Supply Chain as well as Quality, working for small and large Biopharma organizations developing and marketing traditional small molecule products, biologics, and vaccines. A dual EU/US citizen, he’s worked and lived in global roles and locations.
He holds a BS in Mechanical Engineering from Villanova University and an MBA from Northeastern.
David Geoghegan
SVP, Head of Quality Assurance & Validation
Spark Therapeutics

Dr. Pat Yang is one of the most accomplished biotech manufacturing executives and technical operations leaders in the industry.
From 2017 to 2019, Pat was executive vice president at Juno Therapeutics, a leading CAR-T biotech company (now part of BMS) based in Seattle.
Previously, from 2009 to 2013, Pat was Executive Vice President and Global Head of Technical Operations at F. Hoffman-La Roche based in Basel, Switzerland. In this role, he was responsible for Roche’s biopharma process research and development, analytical sciences, engineering, quality, technical regulatory, supply chain and all manufacturing plants with approximately 15,000 employees around the world. From 2003 to 2009, Pat was Executive Vice President of Product Operations at Genentech. He assumed his role as Global Head of the combined technical operations of Roche and Genentech upon the acquisition of Genentech by Roche in 2009.
Before Genentech, Pat worked for 11 years at Merck & Company from 1992 to 2003, based in New Jersey, in various leadership positions including vice president of Asia Pacific Operations and vice president of Global Supply Chain Management. Prior to joining Merck in 1992, Pat spent 12 years at General Electric, serving in research, engineering, technology, and manufacturing leadership roles with increasing scope of responsibilities.
Pat holds a Bachelor of Science from the National Chiaotung University in Taiwan, a Master of Science from the University of Cincinnati, and a Ph.D. in engineering from Ohio State University.
Pat is a member of the Board of Directors of three public companies, Amyris, Codexis and PharmaEssentia. In addition, he is on the board of several private companies, including Acepodia, AltruBio, Antheia and Sana Biotechnology.
Pat Yang
Vice Chairman & Co-Founder
Resilience


Alison Moore brings to Codexis significant experience as a biotechnology and pharmaceutical executive, including an extensive background in biomanufacturing. She is former Chief Technical Officer of Allogene Therapeutics, a pioneering clinical-stage company advancing CAR-T therapies. Prior to Allogene she spent a total of 20 years at Amgen, most recently as Senior Vice President, Process Development, and including roles in Supply Chain and Manufacturing. Dr. Moore also has experience at Genentech as a Director in Chemistry, Manufacturing and Controls, and Regulatory Affairs. Dr. Moore holds a bachelor’s degree in Pharmacology with Honors and a Ph.D. in Cell Biology from Manchester University, England.
Alison Moore
Chief Technical Officer
Codexis

Tongtong joined Genentech in 2018 as the head of Pharmaceutical Technical Development for US Biologics, and was named global head of Pharma Technical Development in April 2021. Prior to her role at Genentech, Tongtong held several scientific, managerial and strategic roles at Eli Lilly, with increasing responsibilities in the Bioproduct Research and Development organisation. Tongtong also worked at Corixa Corporation, a biotech company, where she gained valuable experiences both as a discovery scientist and as a CMC leader in support of therapeutic antibody and cancer vaccine platforms.
Tongtong received her B. Sc. in Molecular Biology from University of Science and Technology of China, a doctorate in Biology from Syracuse University, and completed her postdoctoral training in cell biology and yeast genetics at Cornell University.
Tongtong Wang
Senior Vice President, Global Head Technical Development
Roche

"*" indicates required fields
By completing and submitting this form, you agree to receive marketing emails from Executive Platforms Inc. You can opt-out at any time by utilizing the unsubscribe link provided at the bottom of each email. All data collected will be handled in accordance with our Privacy Policy and Terms of Use.
The Biomanufacturing World Summit 2024 gathered top biomanufacturing leaders to share new ideas and collaborate on solutions that inspired a collective vision for change, shaping the future of the industry.
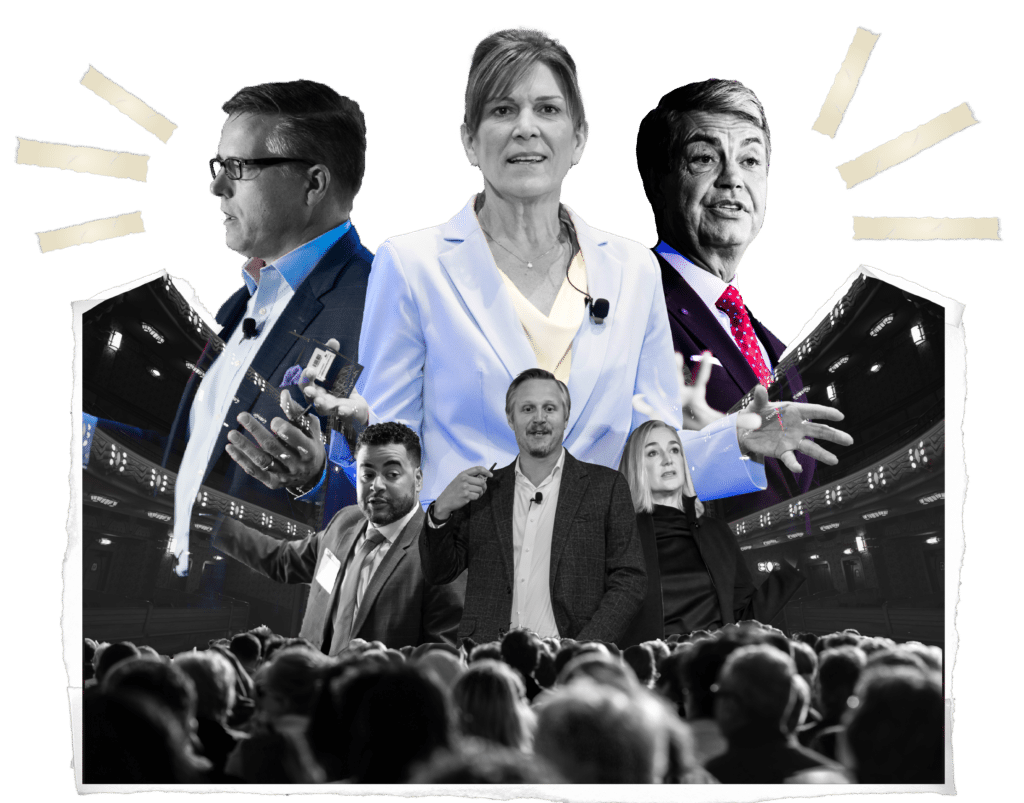
Manufacturing Director, Exact Sciences
VP, Manufacturing, Atara Biotherapeutics
AVP, Head of Manufacturing, Sanofi
CTO, ACELYRIN
Head of Technology Management, mAb, Daiichi Sankyo
Director – CMC Strategy, Bristol-Myers-Squibb
Manufacturing Director, Exact Sciences
VP, Manufacturing, Atara Biotherapeutics
AVP, Head of Manufacturing, Sanofi
CTO, ACELYRIN
































































"*" indicates required fields
By completing and submitting this form, you agree to receive marketing emails from Executive Platforms Inc. You can opt-out at any time by utilizing the unsubscribe link provided at the bottom of each email. All data collected will be handled in accordance with our Privacy Policy and Terms of Use.
Notifications